Changing lives through
innovation
SNAPSHOT
Building a track record of success
What We Do
Virtici provides the leadership, space and funding support to transform innovations into products...
VALUE CREATION
-
1 Select
-
2 Innovate
-
3 Develop
Virtici leadership brings decades of experience
that intersect innovation and product development.
Areas Of Innovation
Virtici is actively seeking the most innovative product and
platform concepts in the following disease areas.
-
Oncology
-
Microbiome
-
Neurology
-
Autoimmunity
-
Inflammation
-
Infectious Disease

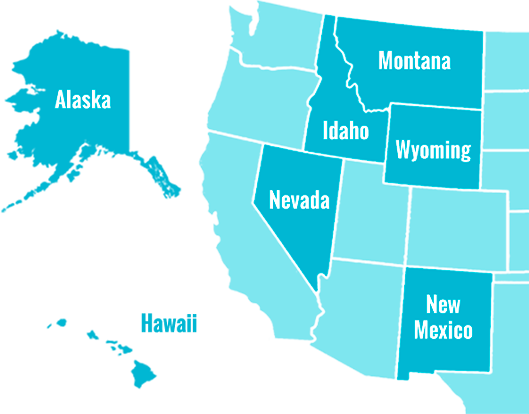
The ASCEND Hub is a Regional Technology Accelerator Hub for the seven Western States: Alaska, Hawaii, Idaho, Montana, Nevada, New Mexico, and Wyoming. The NIH funded program empowers innovators to accelerate biomedical commercialization through entrepreneurial education, mentorship, and technology development funding.
Latest News
FDA Clears Rise Therapeutics’ IND Application to Initiate a Phase 1 Clinical Study of Its Novel Oral Immunotherapy for the Treatment of Rheumatoid Arthritis
Rockville, Maryland – Rise Therapeutics, a biotechnology company engaged in developing novel oral immunotherapeutics, today announced that the U.S. Food and Drug Administration (FDA) has accepted its investigational new drug (IND) application to proceed with a rheumatoid arthritis Phase 1 clinical trial for its program candidate, R-2487. This is Rise Therapeutics’ second clinical program developed using synthetic biology and its proprietary oral biologics delivery platform.
Read moreTEDCO Announces Investment into Rise Therapeutics
TEDCO, Maryland’s economic engine for technology companies, announced a recent Seed Funds investment of $200,000 into Rise Therapeutics, a Maryland-based biotechnology company. TEDCO’s Seed Funds invest in early-stage, technology and life sciences companies and provides access to gap financing.
Read moreFunding Announcement for Novel Synthetic Biology Drug Candidate for Acute Respiratory Distress Syndrome, Developed by Rise Therapeutics
Rockville, Maryland - Rise Therapeutics, a biotech company focused on developing novel immunological-directed synthetic biology-based therapeutics, announced today that it has received funding from the National Institutes of Health (NIH) to develop a novel therapeutic for Acute Respiratory Distress Syndrome (ARDS). The funding will support completion of product validation studies and preparation for clinical development for the program.
Read moreRise Therapeutics Announces FDA Clearance of its IND Application to Initiate a Phase 1 Study of an Oral Immunotherapy for the Treatment of Ulcerative Colitis
Rockville, Maryland, January 31st, 2023 – Rise Therapeutics, a biopharmaceutical company engaged in developing novel oral synthetic biology medicines, today announced that the U.S. Food and Drug Administration (FDA) has accepted its investigational new drug (IND) application to proceed with a Phase 1 clinical trial of its lead program candidate, R-3750.
Read more
Celdara Medical Receives Funding to Develop Broad-Spectrum Antimicrobials for Chronic Lung Infection
Lebanon, NH: The National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) has awarded Celdara Medical, LLC a Phase I Small Business Innovation Research (SBIR) grant for development of broad-spectrum antimicrobial peptides to combat chronic lung infection in patients with cystic fibrosis (CF).
Read more
Virtici Announces Funding from NIH to Develop a New Treatment for Periodontal Disease
Seattle, Washington - Virtici LLC announced today that the National Institute of Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) has awarded a Small Business Innovation Research (SBIR) award to advance VTC-886, a first-in-class small molecule for the prevention of P. gingivalis infection.
Read more